Renal Activity Index for Lupus (RAIL)
January 21, 2026
I. Introduction: A New Paradigm in Monitoring Lupus Nephritis
Lupus Nephritis (LN) is one of the most severe complications of Systemic Lupus Erythematosus (SLE), affecting approximately 50% of patients[1,2]. It is a major determinant of long-term health, with roughly 10% to 22% of those affected progressing to end-stage kidney disease (ESKD)[3]. Effective management requires doctors to accurately measure current kidney inflammation to guide therapy. Historically, this has required invasive procedures, but the Renal Activity Index for Lupus (RAIL) has emerged as a non-invasive "liquid biopsy" designed to assess kidney disease activity through a simple urine sample[4,5].
II. The Problem with Traditional Monitoring
Accurately measuring kidney inflammation is traditionally difficult due to the limitations of existing tools:
The Kidney Biopsy: While the "gold standard" for diagnosis[2], repeat biopsies are invasive, costly, and carry procedural risks[6], making them impractical for routine, frequent monitoring.
Standard Lab Tests: Common measures proteinuria, urinalysis and sediment or complement levels and anti-dsDNA antibodies have limited value and are not well suited to monitor LN accurately[7–9].
The "Molecular Silence Paradox": The standard clinical index used by doctors (rSLEDAI) can be misleading. Studies show that 50% of patients who appear to be in clinical remission (a score of 0) still have significant renal inflammation at the molecular level that traditional tests fail to see[1].
III. How the RAIL Test Works
RAIL is a composite score calculated from a single random urine sample. Instead of relying on a single marker like overall protein levels, RAIL measures six specific protein biomarkers that each reflect a different aspect of kidney health[3,10]. These biomarkers are biological "signposts" that indicate different types of kidney cell injury and inflammation[5].
NGAL: A marker of acute inflammation and injury to the kidney's filtering tubes[11,12].
KIM-1: Specifically signals damage to the proximal tubules[13,14]
MCP-1: A chemical signal that attracts inflammatory immune cells to the kidney[13,14].
Adiponectin: A protein that helps regulate inflammation and may predict long-term damage[13,14].
Hemopexin: An acute-phase reactant associated with tissue damage[3,13].
Ceruloplasmin: An antioxidant protein that increases during the body's acute inflammatory response[3,13].
These biomarkers are produced locally within the kidney tissue, meaning their presence in the urine provides a direct "window" into the organ's internal state.
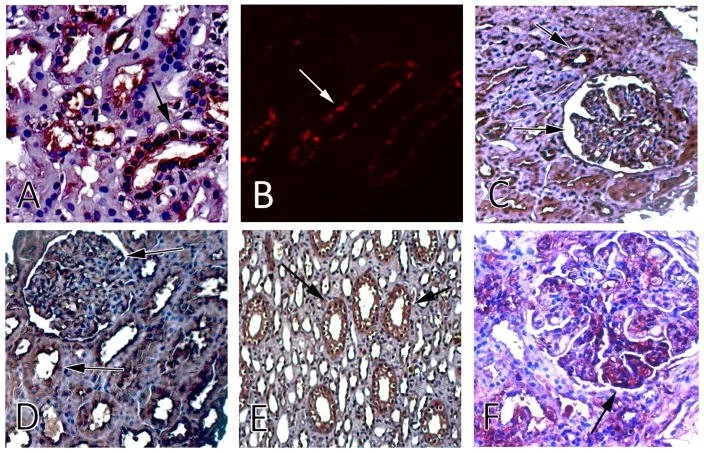
Images show kidney tissue stained to highlight different RAIL biomarkers. Dark or glowing areas mark where inflammation‑related proteins appear in kidney filters and tubules, helping researchers see how active kidney inflammation may be[3].
IV. Accuracy and Customization by Age
Because children and adults have different immune profiles, the RAIL test uses different mathematical formulas (algorithms) for different age groups.
Pediatric RAIL (P-RAIL): In children and young adults, this version is over 92% accurate in identifying high levels of kidney inflammation[13].
Adult RAIL (A-RAIL): The pediatric formula was only "fair" at predicting activity in older adults. Consequently, researchers developed an optimized Adult RAIL algorithm that adjusts the "weights" of the six biomarkers, achieving an excellent accuracy score (AUC 0.88)[3].
V. 2025 Clinical Data: Predicting the Future
Recent major clinical trials (TULIP-LN and ALLURE) have provided groundbreaking data on how RAIL can forecast a patient's treatment course:
Tracking Progress: In the TULIP-LN trial, RAIL scores significantly dropped in patients responding well to treatment. By Week 24, "Complete Responders" saw their scores drop by 2.30 points, while non-responders only saw a 0.88-point decrease[15].
Predicting Remission: Research from the ALLURE trial showed that the RAIL score can predict whether a patient will be in remission at their next follow-up visit with 84–85% accuracy[16].
Identifying Resistance: The 2025 studies suggest that if a patient's RAIL score remains 6.5 or higher, it may objectively indicate that the current treatment is not working or that the patient is struggling with medication adherence[16].
VI. Patent and Commercial Information
The RAIL technology was pioneered by investigators Dr. Hermine Brunner and Dr. Prasad Devarajan at the Cincinnati Children’s Hospital Medical Center[17].
Patent Status: The technology is currently a US Non-Provisional Filing.
Published Patent Applications: US20190317090 and US9880165B2.
Licensing: The technology is listed as an Exclusive License opportunity through Cincinnati Children’s Innovation Ventures.
VII. Current Status and Challenges
Despite its proven accuracy, the RAIL test is not yet part of standard clinical guidelines, such as the 2024 ACR Guideline for Lupus Nephritis[2], which still relies on traditional markers like proteinuria and repeat biopsies. Widespread adoption is currently slowed by the logistical complexity of measuring six proteins at once and the need for a globally standardized, cost-effective testing platform.
VIII. Conclusion
The RAIL test represents a major move toward personalized medicine for lupus. By offering a non-invasive way to see "silent" inflammation and predict treatment outcomes months in advance, it allows clinicians to be proactive rather than reactive[16]. As this technology becomes more widely available, it is poised to help reduce the need for invasive biopsies and improve the long-term kidney health of people living with lupus.
References
Aljaberi N, Wenderfer SE, Mathur A, et al. Clinical measurement of lupus nephritis activity is inferior to biomarker-based activity assessment using the renal activity index for lupus nephritis in childhood-onset systemic lupus erythematosus. Lupus Sci Med. 2022;9(1):e000631. doi:10.1136/lupus-2021-000631
Sammaritano LR, Askanase A, Bermas BL, et al. 2024 American College of Rheumatology (ACR) Guideline for the Screening, Treatment, and Management of Lupus Nephritis. Arthritis Care Res (Hoboken). 2025;77(9):1045-1065. doi:https://doi.org/10.1002/acr.25528
Gulati G, Bennett M R, Abulaban K, et al. Prospective validation of a novel renal activity index of lupus nephritis. Lupus. 2016;26(9):927-936. doi:10.1177/0961203316684212
Romick-Rosendale LE, Brunner HI, Bennett MR, et al. Identification of urinary metabolites that distinguish membranous lupus nephritis from proliferative lupus nephritis and focal segmental glomerulosclerosis. Arthritis Res Ther. 2011;13(6):R199. doi:10.1186/ar3530
Aljaberi N, Bennett M, Brunner HI, Devarajan P. Proteomic profiling of urine: implications for lupus nephritis. Expert Rev Proteomics. 2019;16(4):303-313. doi:10.1080/14789450.2019.1592681
Kang ES, Ahn SM, Oh JS, et al. Risk of bleeding-related complications after kidney biopsy in patients with systemic lupus erythematosus. Clin Rheumatol. 2023;42(3):751-759. doi:10.1007/s10067-022-06394-7
Morales E, Trujillo H, Bada T, et al. What is the value of repeat kidney biopsies in patients with lupus nephritis? Lupus. 2021;30(1):25-34. doi:10.1177/0961203320965703
Gatto M, Radice F, Saccon F, et al. Clinical and histological findings at second but not at first kidney biopsy predict end-stage kidney disease in a large multicentric cohort of patients with active lupus nephritis. Lupus Sci Med. 2022;9(1). doi:10.1136/lupus-2022-000689
Ayoub I, Rovin BH. The Use of Serological Tests in the Care of Patients with Lupus Nephritis. Clinical Journal of the American Society of Nephrology. 2022;17(2). https://journals.lww.com/cjasn/fulltext/2022/02000/the_use_of_serological_tests_in_the_care_of.19.aspx
Cody EM, Rose JE, Huang B, Qiu T, Brunner HI, Devarajan P. Stability of novel urinary biomarkers used for lupus nephritis. Front Pediatr. 2022;Volume 10-2022. https://www.frontiersin.org/journals/pediatrics/articles/10.3389/fped.2022.974049
Brunner HI, Bennett MR, Mina R, et al. Association of noninvasively measured renal protein biomarkers with histologic features of lupus nephritis. Arthritis Rheum. 2012;64(8):2687-2697. doi:https://doi.org/10.1002/art.34426
Suzuki M, Wiers KM, Klein-Gitelman MS, et al. Neutrophil gelatinase-associated lipocalin as a biomarker of disease activity in pediatric lupus nephritis. Pediatric Nephrology. 2008;23(3):403-412. doi:10.1007/s00467-007-0685-x
Bennett MR, Ma Q, Ying J, Devarajan P, Brunner H. Effects of age and gender on reference levels of biomarkers comprising the pediatric Renal Activity Index for Lupus Nephritis (p-RAIL). Pediatric Rheumatology. 2017;15(1):74. doi:10.1186/s12969-017-0202-0
Brunner HI, Bennett MR, Abulaban K, et al. Development of a Novel Renal Activity Index of Lupus Nephritis in Children and Young Adults. Arthritis Care Res (Hoboken). 2016;68(7):1003-1011. doi:https://doi.org/10.1002/acr.22762
Brunner HI, Cody EM, Devarajan P, et al. The Renal Activity Index for Lupus Identifies Active Renal Disease and Treatment Response in Adult Patients With Systemic Lupus Erythematosus and Lupus Nephritis. Arthritis Care Res (Hoboken). 2025;n/a(n/a). doi:https://doi.org/10.1002/acr.25684
O’Connor SK, Devarajan P, Liu J, et al. The Renal Activity Index for Lupus: Validation for Prediction of Kidney Inflammation in Adult Patients with Lupus Nephritis. J Rheumatol. Published online November 15, 2025:jrheum.2025-0504. doi:10.3899/jrheum.2025-0504
Brophy J. Renal Activity Index in Lupus (RAIL) Urinary Biomarkers Predict Treatment Response. https://www.cincinnatichildrens.org/research/support/innovation-ventures/technologies/2016-0606.